1. Influence of CO2 partial pressure on primary metabolism in photosynthetic organisms
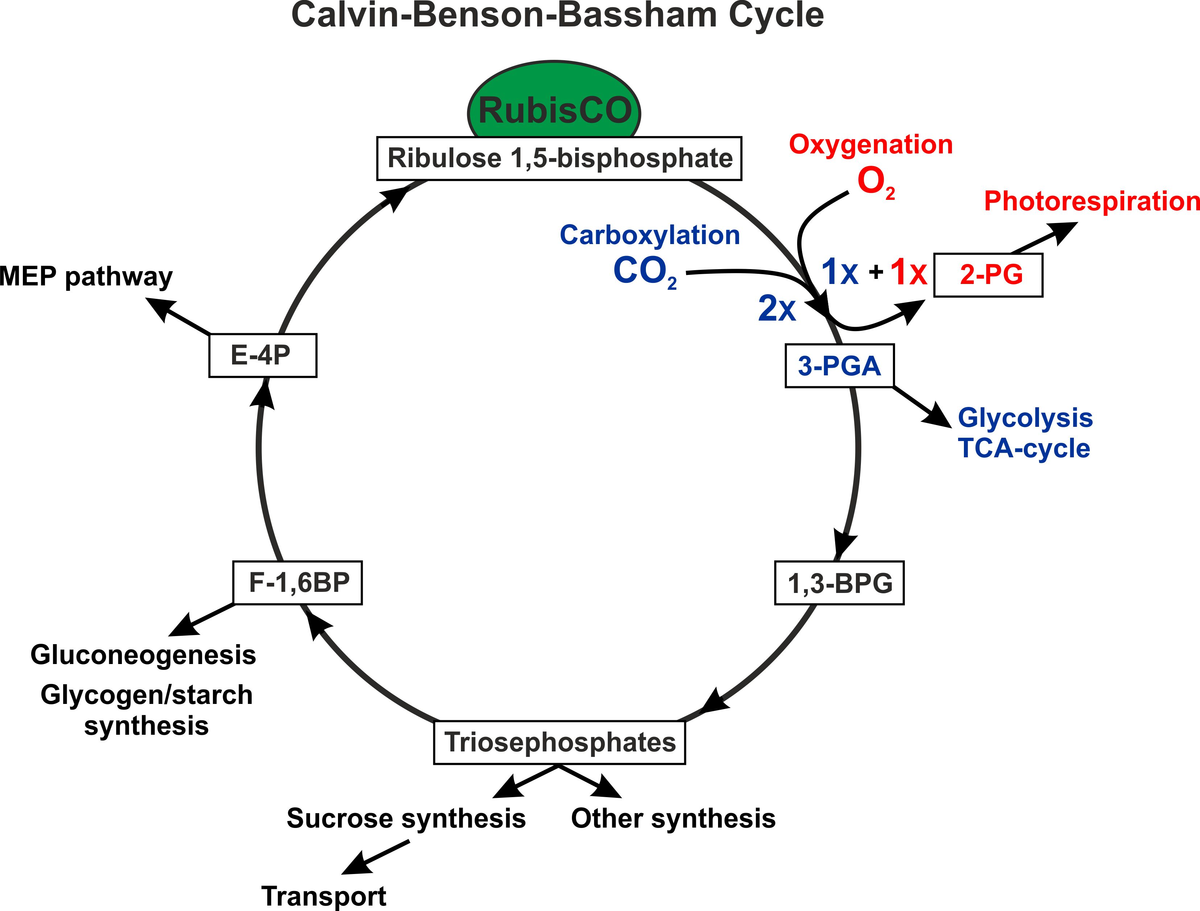
The photosynthetic CO2 fixation via the Calvin-cycle is the central part of primary carbon metabolism in all photoautotrophic organisms. Intermediates of this cycle are used for the synthesis of all other organic compounds. Mainly triosephosphate is exported from the cycle to build up other organic compounds such as sucrose. Photosynthetic carbon is also used as carbon skeleton for the nitrogen assimilation. Another example is erythrose 4-phosphate (E-4P), which serves as major precursor for aromatic amino acids and cell wall biosynthesis. Finally, excess organic carbon is exported as fructose 1,6-bisphosphate (F-1,6BP) and then stored as starch (plants) or glycogen (cyanobacteria), which are used during the night. For these purposes, the Calvin-cycle is highly intertwined with other pathways, such as glycolysis, the oxidative pentose-phosphate and methylerythritol phosphate (MEP) pathways, the tricarboxylic acid (TCA) and GS-GOGAT cycles, photorespiratory 2-PG metabolism, gluconeogenesis, etc. This complex network is influenced by external conditions, which demand different fluxes through the metabolic routes.
During the last years, we analyzed the CO2-dependent alterations of carbon partitioning in the model cyanobacterium Synechocystis sp. PCC 6803. Switches from high-to-low CO2 conditions not only change the activity of the CCM (Burnap et al. 2015) but have also marked influence on the carbon partitioning. Under excess CO2, a large fraction of organic carbon is channeled via triosephosphate into the gluconeogenesis leading to the accumulation of glycogen. After a switch to low CO2 (ambient air) in constant light, photosynthetic activity declines and organic carbon is now mostly exported as 3-PGA into glycolysis and TCA cycle to sustain nitrogen assimilation and other biosynthetic activities. In the transition time, glycogen is consumed in the light to support the demand of other biosynthetic routes. Thus, the response to low CO2 conditions in continuous light resembles the autotrophy to heterotrophy switch in cyanobacteria after exposure to darkness. It has been shown that this CO2-regulated shift is mainly regulated at biochemical level in Synechocystis (Jablonski et al. 2016). The underlying mechanisms are currently analyzed in the research consortium SCyCode (FOR2816).
Our work on the model plant Arabidopsis thaliana showed that transition from high-to-low CO2 had also marked impact on the primary carbon and nitrogen metabolism. Most remarkably, the flux through the photorespiratory 2-PG metabolism was immediately activated, which is not involving the activation of gene expression for enzymes involved in photorespiration (Eisenhut et al., 2015). Interestingly, the metabolic changes detected in high-to-low CO2 shifted Arabidopsis leaves are similar to cyanobacteria, which implies that these evolutionary related organisms not only possess a conserved metabolism but also similar flux control mechanisms (Timm et al. 2012a, Orf et al. 2016). The influence on flux control by specific enzymes in the photorespiratory 2-PG metabolism has been analyzed in attempts to down- or upregulate specific photorespiratory enzymes in Arabidopsis. One main finding here was the observation that an increased photorespiratory flux due to overexpression of the H- or L-protein, which are involved in the mitochondrial part of plant photorespiratory 2-PG metabolism, improved photosynthesis and growth of plants (Timm et al. 2012b, 2015). Currently, we are analyzing how photorespiratory and photosynthetic pathways interact and how these interactions are regulated in a changing environment.
References
Burnap RL, Hagemann M, Kaplan A (2015) Regulation of CO2 concentrating mechanism in cyanobacteria. Life 5:348-371
Eisenhut M, Bräutigam A, Timm S, Florian A, Tohge T, Fernie A, Bauwe H, Weber APM (2016) Photorespiration is crucial to the dynamic response of photosynthetic metabolism and stomatal movement to altered CO2 availability. Molecular Plant 10:47-61
Jablonsky J, Papacek S, Hagemann M (2016) Different strategies of metabolic regulation in cyanobacteria: from transcriptional to biochemical control. Scientific Reports 6:33014
Orf I, Timm S, Bauwe H, R. Fernie AR, Hagemann M, Kopka J, Nikoloski Z (2016) Can cyanobacteria serve as a model of plant photorespiration? – A comparative analysis of metabolite profiles. Journal of Experimental Botany 67:2941-2952
Timm S, Mielewczik M, Florian A, Frankenbach S, Dreissen A, Hocken N, Fernie AR, Walter A, Bauwe H (2012a) High-to-low CO2 acclimation reveals plasticity of the photorespiratory pathway and indicates regulatory links to cellular metabolism of Arabidopsis. PLoS ONE 7:e42809
Timm S, Florian A, Arrivault S, Stitt M, Fernie AR, Bauwe H (2012b) Glycine decarboxylase controls photosynthesis and plant growth. FEBS Letters 586:3692-3697
Timm S, Wittmiß M, Gamlien S, Ewald R, Florian A, Frank M, Wirtz M, Hell R, Fernie AR, Bauwe H (2015) Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. The Plant Cell 27:1968-1984