2. Sensing fluctuating CO2 partial pressure
Photosynthetic CO2 assimilation is highly intertwined with the primary carbon as well as nitrogen metabolism and needs to respond towards changing environmental conditions. In this regard it is essential for photoautotrophic organisms to sense the availability of CO2 to change carbon fluxes accordingly.
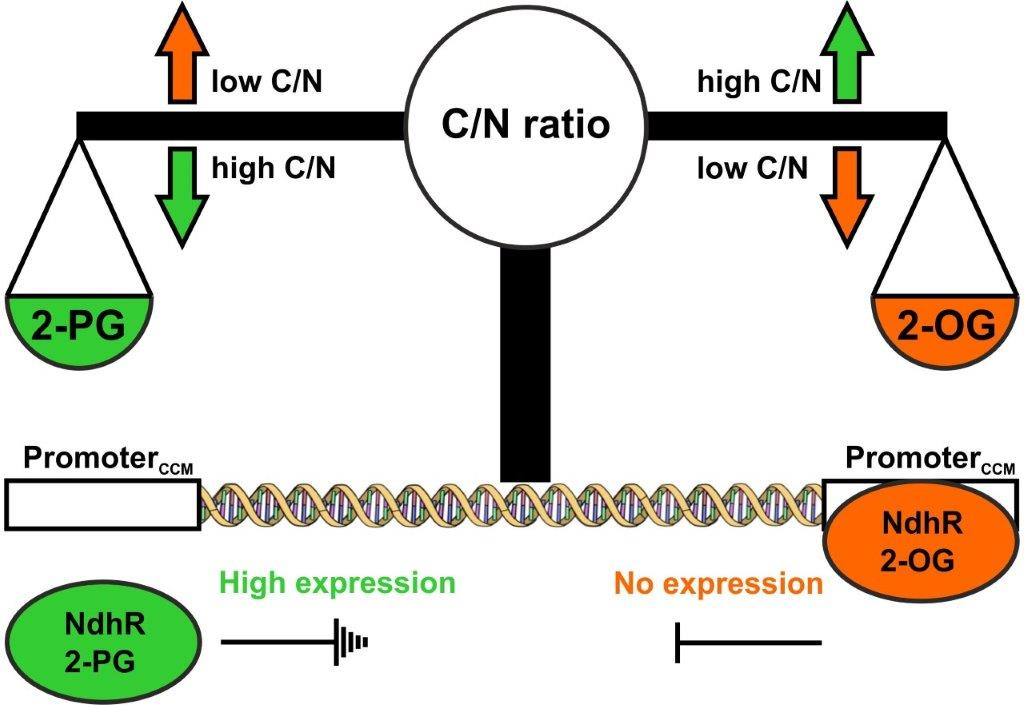
The cyanobacterium Synechocystis sp. PCC 6803 adjusts the activity of the CCM under different CO2 conditions mainly on the transcriptional level. The transcriptional factor NdhR has been shown to act as the main repressor of the CCM under high CO2 conditions, while it becomes inactivated when CO2 is limiting. The DNA-binding activity of NdhR depends on metabolic signals responding to the external CO2 amount. The repressor is activated by binding of the TCA cycle intermediate 2-oxoglutarate (2-OG), which accumulates under excess of CO2, while the repressor is inactivated upon binding of the photorespiratory intermediate 2-PG, which accumulates under CO2-limiting conditions (Zhang et al. 2018). However, mutation of ndhR only abolished parts of the low CO2 response in Synechocystis (Klähn et al. 2015), which clearly implies the involvement of more factors and signals. One additional transcriptional factor CyAbrB2 has been described, which cooperates with NdhR in the low CO2 response of Synechocystis (Orf et al. 2016).
Recently, a protein called SbtB that shows structural similarities with the nitrogen-sensing protein PII has been identified as novel player in the CO2 response of cyanobacteria (Selim et al. 2018). SbtB is binding the secondary messenger cAMP, which has been before suggested to act as signal molecule in carbon sensing among bacteria and eukaryotes including humans. The SbtB-based network in cAMP-mediated CO2 sensing is currently analyzed in cooperation with the group of Prof. Karl Forchhammer (University of Tübingen) in the frame of the priority program SPP 1879. Moreover, previously not annotated small proteins have been recently identified in Synechocystis (Baumgartner et al. 2016), which might play a role in the metabolic regulation of cyanobacteria. First data obtained by us within the priority program SPP2002 suggest that the small proteins Norf4 and AcnSP regulate the activities of glycolysis and TCA cycle, respectively.
The sensing of CO2 in the model plant Arabidopsis involves also the metabolic signal 2-PG, which has been shown in plant with lowered or accelerated expression of the photorespiratory enzyme PGPase. One major observation was that the efficient conversion of 2-PG is key to maintain optimal Calvin-cycle activity and the export of carbon towards starch (Flügel et al. 2017). Fluctuations in the cellular 2-PG occur under short- and long-term environmental stresses, hence, its sensing could serve as signal for the acclimation of plant metabolism (Timm et al. 2019). In addition, redox control of enzymes in the mitochondrial steps of photorespiration is involved in adjusting the carbon flux according to the CO2-regulated photosynthesis in plants (Reinholdt et al. 2019, Fonseca-Pereira et al. 2020). The control of the mitochondrial L-protein by thioredoxins has been identified as main redox-controlled step, given this enzyme plays an important role in four major enzyme complexes in mitochondria. In collaboration with Prof. Danilo M. Daloso (University of Fortaleza, Brazil) and Prof. Alisdair R. Fernie (Max-Planck Institute for Molecular Plant Physiology, Golm) we are currently investigating how these mechanism controls carbon fluxes through the photorespiratory 2-PG metabolism, the TCA-cycle, and the degradation of branched chain amino acids.
References
Baumgartner D, Kopf M, Klähn S, Steglich C, Hess WR (2016) Small proteins in cyanobacteria provide a paradigm for the functional analysis of the bacterial micro-proteome. BMC Microbiology 16:285
Flügel F, Timm S, Arrivault S, Florian A, Stitt M, Fernie AR, Bauwe H (2017) The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in Arabidopsis. The Plant Cell 29:2537-2551
Fonseca-Pereira P, Souza PVL, Hou LY, Schwab S, Geigenberger P, Nunes-Nesi A, Timm S, Fernie AR, Thormählen I, Araújo WL, Daloso DM (2019) Thioredoxin h2 contributes to theredox regulation of mitochondrial photorespiratory metabolism. Plant Cell and Environment 43:188-208
Klähn S, Orf I, Schwarz D, Matthiessen JKF, Kopka J, Hess WR, Hagemann M (2015) Integrated transcriptomic and metabolomic characterization of the low-carbon response using an ndhR mutant of Synechocystis sp. PCC 6803. Plant Physiology 169:1540-1556
Orf I, Schwarz D, Kaplan A, Kopka J, Hess WR, Hagemann M, Klähn S (2016) CyAbrB2 contributes to the transcriptional regulation of low CO2 acclimation in Synechocystis sp. PCC 6803. Plant Cell Physiology 57:2232-2243
Reinholdt O, Schwab S, Zhang Y, Reichheld JP, Fernie AR, Hagemann M, Timm S (2019) Redox-regulation of photorespiration through mitochondrial thioredoxin o1. Plant Physiology 181:442-457
Selim KA, Haase F, Hartmann MD, Hagemann M, Forchhammer K (2018) The PII-like signaling protein SbtB links cAMP sensing with cyanobacterial inorganic carbon response. Proceeding of the National Academy of Sciences USA 115:E4861-E4869
Timm S, Woitschach F, Heise C, Hagemann M, Bauwe H (2019) Faster removal of 2-Phosphoglycolate through photorespiration improves abiotic stress tolerance of Arabidopsis. Plants (MDPI) 8:563
Zhang CC, Zhou CZ, Burnap RL, Peng L (2018) Carbon/nitrogen metabolic balance: Lessons from cyanobacteria. Trends in Plant Sciences 23: 1116-1130

